- Document History
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report to a Moderator
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report to a Moderator
DISEASEKG
EVAN LYNCH, JIAJIA LUO, ELLIOT HWANG, JORDAN KLEIN
INTRODUCTION
For our final project, we aimed to show proof‐of‐concept design for a device which can
analyze EKG data obtained from a low drift, 2‐lead circuit design and accurately diagnose
several heart conditions. We looked to provide an easy‐to‐use system, that’s at low cost while
still retaining at least 90% accuracy. The DiseasEKG system works by placing 2‐leads on the
patient’s torso, each just below the pectoral muscle, and running those wires to a circuit, which
is subsequently run to our LabVIEW VI via a DAQ board. Our circuit would need to amplify EKG
signal while using a band‐pass filter to better hone in the correct range of frequencies, and also
using an innate notch filter to filter out waveform noise. The LabVIEW VI will acquire and
analyze signals by interfacing both Matlab and LabVIEW script. Essentially, our device looks to
decrease the amount of time, and abate some of the inaccuracy associated with EKG disease
detection. Further applications of our device include bedside cardiology training, as well as
providing further market advantages than current EKG machines.
EXPERIMENTAL SETUP
As previously mentioned, we utilized a low drift, 2‐lead circuit design, as opposed to the
standard 12‐lead circuit utilized in hospitals today. We were hoping to maintain the accuracy in
EKG signal while reducing the complexity associated with the 12‐lead system. In order to reduce
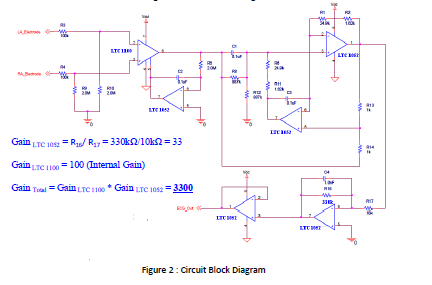
DC drift, we obtained low‐drift amplifiers from Linear Technologies—LTC 1100, and LTC 1052.
LTC 1100 is an instrumentation amplifier with a built‐in gain of 100, while LTC 1052 is an
operational amplifier used to construct our filtering system, and with a gain associated with
R16/ R17.Through utilizing these amplifiers, we were able to acquire a significantly more stable
signal, as compared to our 3‐lead EKG circuit utilizing AD620 and LM741. These amplifiers cost
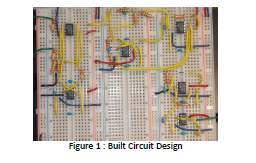
between $3‐5 per amplifier, but DiseasEKG was able to obtain samples for free. A picture of our
built circuit can be seen in Figure 1. Note: Green wires are I/O wires, red and blue are positive
and negative power supply, respectively. Black is ground and yellow is connecting wires. A
picture of our circuit block diagram, with resistor values and total gain, can be seen in Figure 2.
While the launching pad for our unique circuit design was courtesy of
openecgproject.org[1] and their proposed block diagram, our LabVIEW design was carefully
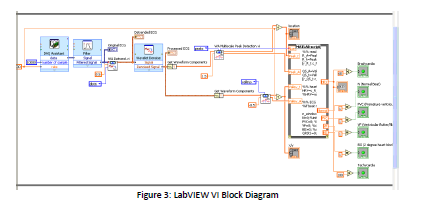
thought‐out and experimentally‐derived. Once the signal was obtained, we used a Notch‐filter
in LabVIEW to further filter out waveform noise. After waveform noise was filtered, the data
was again denoised and detrended using internal LabVIEW programs, to further reduce DC drift
and wavelet noise. The output was dubbed ‘Processed Signal,’ and sent to the front panel for
doctor analysis. From the Processed Signal, we used peak detectors to determine the location
of the peaks, and the amplitude of the peaks, for disease identification. Then, with some help
from literature, we interfaced novel Matlab code to analyze the data and determine disease
origination for 3 diseases: Ventricular Flutter, 2° Heart Blockage, and Premature Ventricular
Contraction[2][3]. Additionally, our design was able to discover if a patient’s heart rate was too
high (tachycardia) or too low (bradycardia), so ideally this design would be utilized under
resting conditions. A picture of both the front panel and block diagram can be seen below.
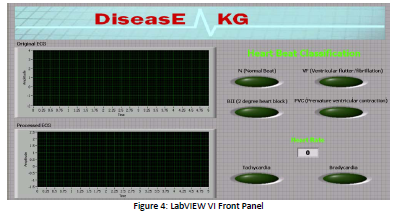
RESULTS AND DISCUSSION
While sampling of our results did not meet desired expectations, all results returned
positive outlooks for the future. Our device did show proof‐of‐concept, as our results returned
5/6 normal results, and did actually capture an inconsistency in another individual not
previously noticed before. While the individual did not show characteristics of the three
diseases we were examining, he did however produce signal that the system recognized as
atypical, which is the first step in validating our bedside design. Figure 5 highlights our findings.
Some of the achieved acceptance criteria consisted of obtaining stable signal quickly and easily,
and at a low cost. However, as referenced earlier, we were not able to test the one technical
acceptance criteria, 90% accuracy, due to inability to obtain willing parties to test our system.
However, we are confident that in future testing, this device would prove robust and accurate
upwards of 90%.

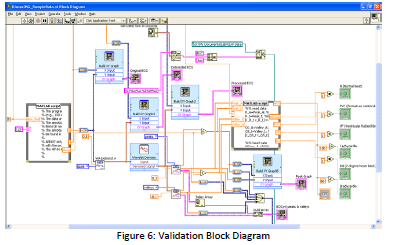
In order to test our devices accuracy in diagnosing the diseases we targeted, we
developed an additional LabVIEW VI which would take signal from the MIT‐BIH database of EKG
signals[4] and analyze the heart signals for relevant heart conditions. This VI is completely similar
to the bedside design, except for utilizing Matlab code to build a waveform graph in LabVIEW
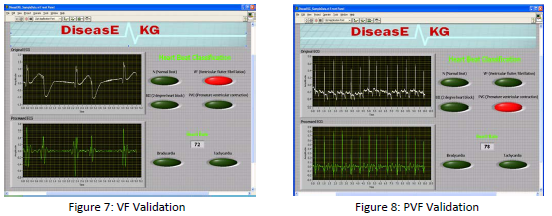
from the database waveforms. A picture of our validation VI is pictured below, with the
confirmation of two of our diseases. We were unable to validate the 2° Heart Blockage due to
memory constraints on the system.
C
ONCLUSIONS & APPLICATION
Our experiment shows that disease identification via an EKG machine is a viable option.
However, our results are not yet statistically significant, and additionally, the project is rather
raw even at its completion. We only discuss 3 diseases, and the Matlab code, and literature
research required to identify and isolate these diseases required 4 weeks of constant work
beyond classroom parameters. Since isolation of many diseases’ EKG signal characteristics is
poorly documented in general, let alone for a 2‐lead system, expansion of this device beyond
our parameter could increase cost and decrease accuracy, but neither of these considerations
can be confirmed, only assumed. While this device would be immensely helpful in life‐saving
diagnosis of heart irregularities, the practicality of expansion beyond our device could be
drastically‐reduced without close collaboration with top cardiologists, and advanced
programmers.
However, that is not to say this device is not possible—in fact—we believe we’ve proved
that this type of diagnosis can be achieved and has use. In a hospital setting, we believe that
this device could potentially aid in the teaching of new cardiologists, as well as improving
diagnosis of cardiologists everywhere. Additionally, we believe that the easy‐to‐read interface
could extend use beyond cardiologists to hospital doctors and nurses at bedside. Improvements
on the device include increased disease detection, and interface with wrist‐electrode design as
opposed to chest‐electrode design for easier application to patients. Also, the system requires a
10‐second interval of signal before accurate diagnosis—we hope to reduce this in the future.
Another concern is LabVIEW seems to utilize its usable memory rather quickly, as the number
of samples required for accurate detection is rather high. As LabVIEW inputs and writes these
samples, the amount of time we can take EKG signal seems to be only about 1 minute of clear
signal. We hope that we can fix this issue in the future. Further, if the system enters the market,
we would look to increase portability of the design for potential use in multiple rooms, as well
as the possibility for ambulance use. Some sources of error are: human error, leads picking up
muscle activity, memory constraints and lag time skewing results.
ATTACHMENT: ORIGINAL REPORT
